- INTRODUCTION
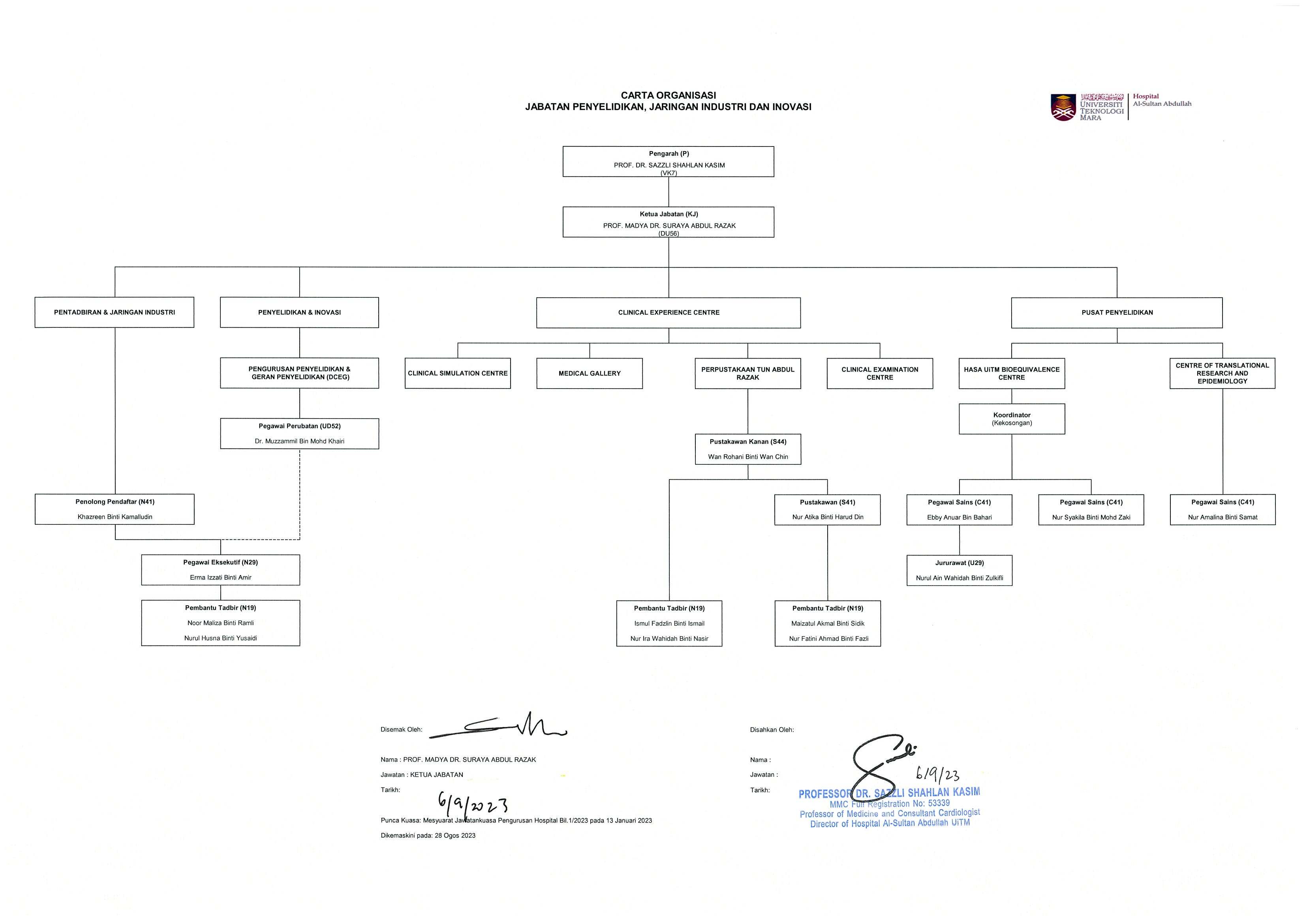
- ORGANISATION CHART
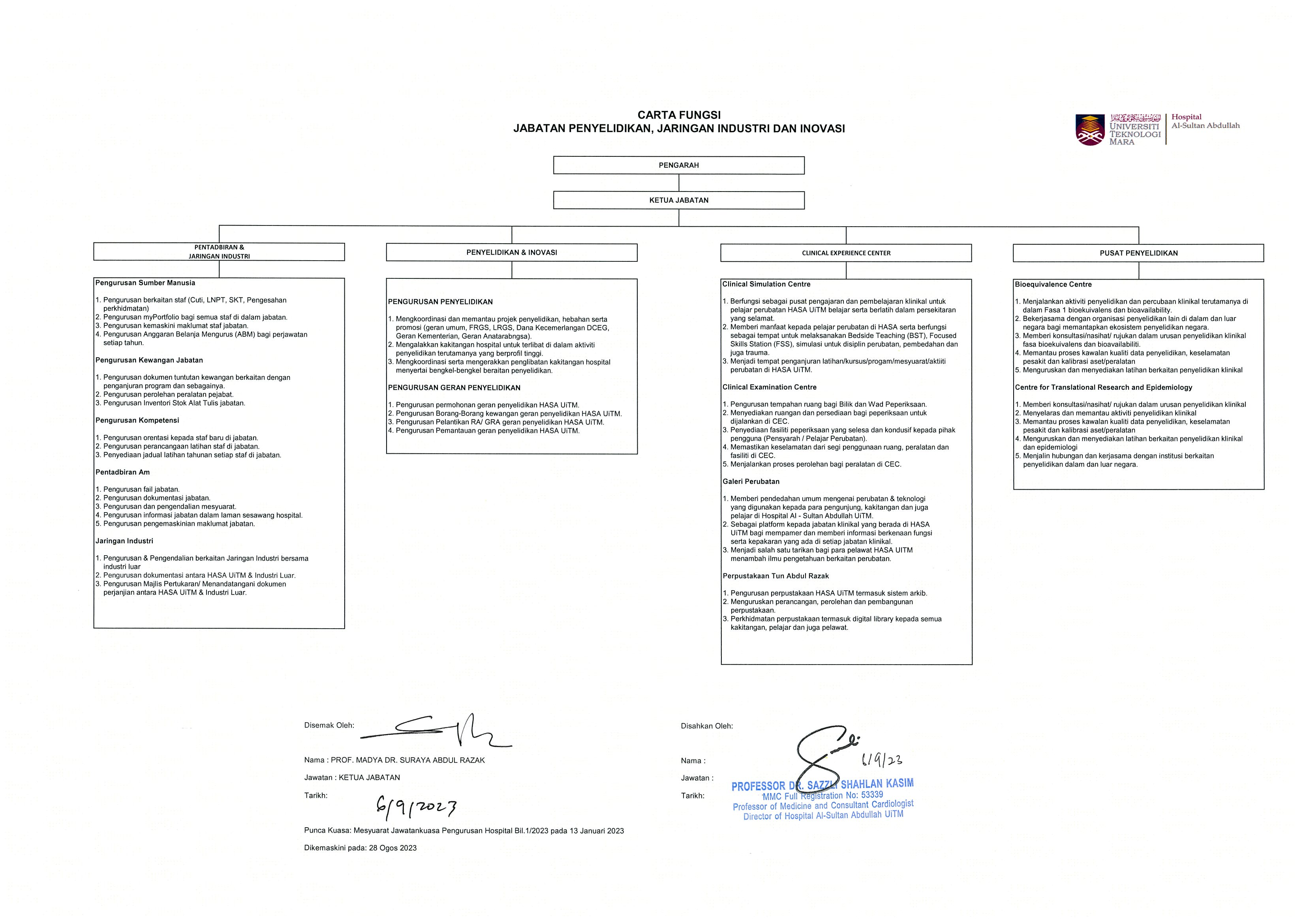
- FUNCTIONAL CHART
- VISION & MISSION
- OBJECTIVE
- SERVICES
- OPERATIONAL HOUR
- STAFF DIRECTORY
- DOWNLOAD
- NEWS @ PJI
- POLICY AND PROCEDURE
Welcome ToDepartment of PJIResearchIndustrial LinkagesInnovation| HASA UiTM
The Department of Research, Innovation, and Industrial Linkages (PJI) aims to strengthen the research and innovation culture as well as the industrial networking of HASA UiTM. The research culture shall be empowered to improve the quality of healthcare and enable HASA UiTM to be a premier hospital that makes aspects of research, innovation, and industry networking one of the cores in ensuring the provision of effective and excellent services.
At the same time, HASA UiTM also believed in enrichment of education and PJI through the library (PTAR) and various Clinical Experience Centre programmes committed towards these efforts. Finally, PJI also currently involved in the management and the administration of UiTM’s Cardio Vascular and Lung Research Institute (CaVaLRI) as well 2 clinical trials unit which are HUiTM Bioequivalence Centre (BE Centre) and Centre For Translational Research and Epidemiology (CenTRE).


| Research and Innovation | Dana Clinical Excellence Grant (DCEG) |
| Digital Health Unit | |
| Innovation | |
| Industrial Linkages | Memorandum of Understanding |
| Memorandum of Agreement | |
| Memorandum of Collaboration | |
| Non-Disclosure Agreement | |
| Letter of Intent | |
| Research Agreement/Research Collaboration Agreement | |
| Research Centre | HASA UiTM Bioequivalence Centre (BE Center) |
| Centre for Translational Research and Epidemiology (CenTRE) | |
| Tun Abdul Razak Library (PTAR) | Administrative and Archive Unit |
| Planning and Development | |
| Services Unit | |
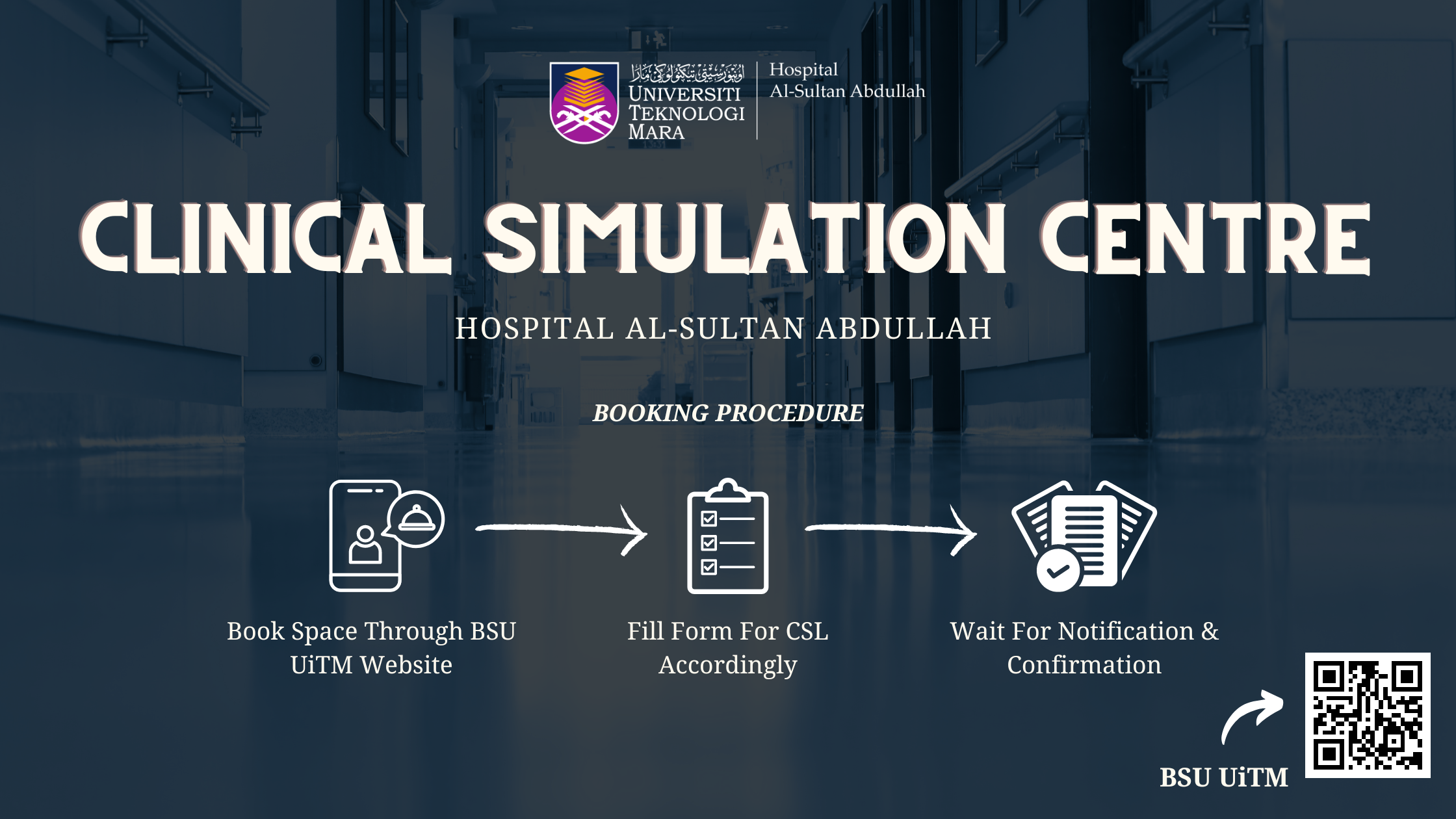
| Clinical Experience Centre | Clinical Skills Centre |
| Medical Gallery |
OFFICIAL ADDRESS |
Department of Research, Industrial Linkages & Innovation Level 5, Hospital Al-Sultan Abdullah, UiTM Puncak Alam 42300 Puncak Alam Selangor. |
EMAIL ADDRESS |
pjihuitm@uitm.edu.my |
OPERATIONAL HOURS |
Monday to Friday : 8.00 a.m until 5.00 p.m Saturday, Sunday & Public Holiday: Closed |
 |
: | ASSOC. PROF. DR. SURAYA ABDUL RAZAK |
| : | HEAD OF RESEARCH, INDUSTRIAL LINKAGES AND INNOVATION DEPARTMENT | |
| : | 03-3396 3000 (ext: 14501) |
|
| : | suraya617@uitm.edu.my |
 |
: | DR. MUZZAMMIL MOHD KHAIRI |
| : | MEDICAL OFFICER (UD52) | |
| : | 03-3396 3000 (ext: 14507) |
|
| : | muzzammilkhairi@uitm.edu.my |
 |
: | PN. WAN ROHANI WAN CHIN |
| : | SENIOR LIBRARIAN (S44) | |
| : | 03-3396 3000 (ext: 12801) |
|
| : | rohani4029@uitm.edu.my |
 |
: | PN. KHAZREEN KAMALLUDIN |
| : | ASSISTANT REGISTRAR (N41) | |
| : | 03-3396 3000 (ext: 14509) |
|
| : | khazr635@uitm.edu.my |
 |
: | EN. EBBY ANUAR BAHARI |
| : | SCIENCE OFFICER (C41) | |
| : | 03-3396 3000 (ext: 14508) |
|
| : | ebbyanuar@uitm.edu.my |
 |
: | PN. NUR SYAKILA MOHD ZAKI |
| : | SCIENCE OFFICER (C41) | |
| : | 03-3396 3000 (ext: 14518) |
|
| : | nursya8757@uitm.edu.my |
 |
: | CIK NUR AMALINA SAMAT |
| : | SCIENCE OFFICER (C41) | |
| : | 03-3396 3000 (ext: 14508) |
|
| : | amalinasamat@uitm.edu.my |
 |
: | PN. ERMA IZZATI AMIR |
| : | EXECUTIVE OFFICER (N29) | |
| : | 03-3396 3000 (ext: 3500) |
|
| : | izzati7754@uitm.edu.my |
 |
: | PN. NURUL AIN WAHIDAH ZULKIFLI |
| : | NURSE (U29) |
|
| : | 03-3396 3000 | |
| : | nurulain3480@uitm.edu.my |
 |
: | CIK NOOR MALIZA RAMLI |
| : | CLERK (N19) | |
| : | 03-3396 3000 (ext: 14517) |
|
| : | malizaramli@uitm.edu.my |
 |
: | PN. NURUL HUSNA YUSAIDI |
| : | CLERK (N19) | |
| : | 03-3396 3000 (ext: 14517) |
|
| : | husnayusaidi@uitm.edu.my |
RESEARCH
|
Research Application Checklist |
||
|
Application Form To Conduct Research |
||
|
EHR/HIS Data Request Form |
||
|
Ethics Approval Application Form [REC 2] |
||
|
Study Risk Classification Form [REC 3] |
||
|
Participant Information Form [REC 4] |
||
|
Research Progress Report Form |
||
|
Final Research Report Form |
||
|
Sub - Investigator Application Form |
DANA CLINICAL EXCELLENCE GRANT (DCEG)
VOT11000 : RESEARCH ASSISTANT PAYMENT (RA/GRA)
VOT21000 : TRAVEL, ACCOMMODATION AND TRANSPORTATION
VOT24000 : RENT
VOT27000 : RESEARCH SUPPLIES AND MATERIALS
VOT28000 : MINOR RESTORE AND RENOVATION
VOT29000 : EXPERTISE SERVICES
VOT35000 : ACCESSORIES AND EQUIPMENT
|
Domestic Travel Claim Form |
||
|
Domestic Travel Advance Form |
||
|
Domestic Travel Adjustment Form |
||
|
Miscellaneous Claim Form / Honorarium / Expert Services / Research Assistant Salary Payment |
||
|
Miscellaneous Adjustment Advance Form |
||
|
Local Order Application Form |
||
|
Payment Management Form |
||
|
Conference |
||
|
Research Assistant / Specialist Services Appointment Letter Template |
||
|
Work / Service Verification Form |
||
|
Goods Receipt Certificate Form (Capital Property / Inventory / Supplies) |
RESEARCH AT HASA UiTM
- SERVICES
- START HERE
- RESEARCH ETHICS
- CONDUCTING RESEARCH IN HASA
- APPLICATION DOCUMENT CHECKLIST
- EHR/HIS DATA REQUEST FOR RESEARCH PURPOSES
- DANA CLINICAL EXCELLENCE GRANT (DCEG)
- RESEARCH REPORTING
- PUBLICATION & PRESENTATION
- CONTACT US
- QUESTION / FEEDBACK
ResearchAl-Sultan AbdullahHospital| UiTM
We seek to promote, enable, and empower research culture that conform with the local Good Clinical Practice (GCP) and the international standard. Connect with us today for your every research needs.
1) HASA
for general research/ non clinical research
2) Centre For Translational Research and Epidemiology (CenTRE)
for phase III/IV studies, translational or epidemiological study
3) HASA Bioequivalence Centre (BE Centre)
*coming soonfor phase I/II studies
-updated 14 July 2023-

Independent Ethics Committee (IEC)
Research Ethics Committee UiTM (REC) is UiTM's own Independent Ethics Committee (IEC) that is registered under the Drug Control Authority (DCA). As of 22 March 2023, there are 14 ethics committees registered with DCA. In addition to the main or centralised ethics committee in UiTM located in Shah Alam main campus, all UiTM’s state branches and faculties had enacted their own ethics committee since 2016 in accordance with the Pekeliling Timbalan Naib Canselor (Penyelidikan dan Inovasi) Bilangan 3 Tahun 2016, Penubuhan Jawatankuasa Penyelidikan di Fakulti dan Cawangan. However, HASA is neither a state branch nor faculty and as such is not permitted to establish its own ethics committee. Furthermore, the creation of an ethics committee in HASA is redundant given the specialist and consultants serving HASA are from the Faculty of Medicine UiTM in which the faculty is already equipped with its own faculty research ethics committee.
REC and Faculty of Medicine FREC (if the study deems to be of minimal risk) are primarily responsible to evaluate, approve or reject research applications to be conducted within UITM at the highest level.
For more information on REC UiTM please visit https://www.recuitm.org/.
For more information on Faculty of Medicine FREC please visit https://medicine.uitm.edu.my/index.php/en/research/ecosystem.
REC UiTM 2023 Meeting Calendar.
|
Month |
Meeting ID |
Application Closing Date |
Meeting Date |
|
Jan |
Bil. 1/2023 |
03/01/2023 |
17/01/2023 |
|
Feb |
Bil. 2/2023 |
07/02/2023 |
21/02/2023 |
|
Mac |
Bil. 3/2023 |
07/03/2023 |
21/03/2023 |
|
Apr |
Bil. 4/2023 |
04/04/2023 |
18/04/2023 |
|
May |
Bil. 5/2023 |
02/05/2023 |
16/05/2023 |
|
Jun |
Bil. 6/2023 |
06/06/2023 |
20/06/2023 |
|
Jul |
Bil. 7/2023 |
04/07/2023 |
18/07/2023 |
|
Aug |
Bil. 8/2023 |
01/08/2023 |
15/08/2023 |
|
Sep |
Bil. 9/2023 |
05/09/2023 |
19/09/2023 |
|
Oct |
Bil. 10/2023 |
03/10/2023 |
17/10/2023 |
|
Nov |
Bil. 11/2023 |
07/11/2023 |
21/11/2023 |
|
Dec |
Bil. 12/2023 |
05/12/2023 |
19/12/2023 |
Faculty of Medicine Research Ethics Committee (FREC) 2023 Meeting Calendar.
|
Month |
Meeting ID |
Application Closing Date |
Meeting Date |
|
Jan |
Bil. 1/2023 |
- |
10/01/2023 |
|
Feb |
Bil. 2/2023 |
31/01/2023 |
14/02/2023 |
|
Mac |
Bil. 3/2023 |
28/02/2023 |
14/03/2023 |
|
Apr |
Bil. 4/2023 |
28/03/2023 |
11/04/2023 |
|
May |
Bil. 5/2023 |
18/04/2023 |
09/05/2023 |
|
Jun |
Bil. 6/2023 |
30/05/2023 |
13/06/2023 |
|
Jul |
Bil. 7/2023 |
27/06/2023 |
11/07/2023 |
|
Aug |
Bil. 8/2023 |
25/07/2023 |
08/08/2023 |
|
Sep |
Bil. 9/2023 |
29/08/2023 |
12/09/2023 |
|
Oct |
Bil. 10/2023 |
29/09/2023 |
10/10/2023 |
|
Nov |
Bil. 11/2023 |
31/10/2023 |
14/11/2023 |
|
Dec |
Bil. 12/2023 |
28/11/2023 |
12/12/2023 |
*The calendars served as a guide and are subjected to change by both REC and FREC respectively. PJI will not be held responsible or liable in any way for any claims, damages, losses, expenses, costs or liabilities whatsoever from the usage of this information.
Flowchart 1: Research applications in HASA from various institutions
Flowchart 2: Research application in HASA (UiTM and HASA-level review)
Flowchart 3: Research collaborative process
Flowchart 4: Request for EHR/HIS data extraction for research purposes
Flowchart 5: Publication and Presentation approval
Ethics Application.
Ethics application is done online via UiTM Research Ethics Depository (RED). Non-UiTM personnel may submit their application by choosing the option “guest”. Please choose “Fakulti Perubatan” as the branch of choice for research application evaluation. Research deemed to be more than minimal risk will be evaluated by REC while minimal risk research may be delegated to be reviewed by the Faculty of Medicine FREC. Ethics approval letter is required when applying to conduct research or collect data in HASA.
Institutional Approval.
Principal investigator is required to send a cover/request letter address to HASA director and to fill in Borang permohonan menjalankan penyelidikan di HASA UiTM/PPUiTM. Principal investigator is also required to submit a few REC forms that were submitted via RED during the ethics application processes (see under Document Checklist below).
Courteous reminder that retrospective institutional approval application or conducting research/data collection without institutional approval is improper, unethical and does not conform to the academic and research integrity.
Sub-Investigator and Head of Department Approval.
The principal investigator is also required to elect a minimum of one (1) sub-investigator from HASA who is from the department or discipline related to the research. For example, if the principal investigator would like to conduct research related to critical care nursing, he or she needs to elect a nurse from ICU or other critical care unit to be the sub-investigator. By extension, the principal investigator also needs to obtain permission and approval from the head of the department, in this case, the HOD of the Anaesthesiology department. If you are unsure which department to approach you may contact PJI for recommendation.
Checklist
1) Checklist for research application in HASA
Cover letter & Research Application Form
2) Application to conduct research in HASA/Cover letter (address to HASA director)
3) HASA Research Request Form [(500-PJI (18/4/28)/Borang (01)]
Sub-Investigator & HOD Approval
4) Sub-Investigator and HOD approval form [(500-PJI (18/4/28)/Borang (02)] *Not required for ISR
REC Forms
5) REC 2 Form (Borang Permohonan Kelulusan Etika) with added Gantt chart or equivalent form: FERC/BERC 1(2021), C.F.B.CoE ERC1(2022)
6) REC 3 Form (Borang Klasifikasi Risiko Kajian)
7) REC 4 Form (Borang Maklumat Peserta) or equivalent form: C.F.B.CoE ERC2 (2022)
8) Ethics approval letter from Research Ethics Committee (REC)/ FREC
EHR/HIS Data Request
9) Description of Parameters Needed For Data Collection [(500-PJI (18/4/28)/Borang (03)] *if applicable
10) Clinical Trial Agreement or equivalent MOU/MOA/NDA or HASA EHR Confidentiality Agreement *if applicable
Grant Letter
11) Research grant approval letter *if applicable
Research Processes in HASA.
Request for Electronic Health Records (EHR) data for research.
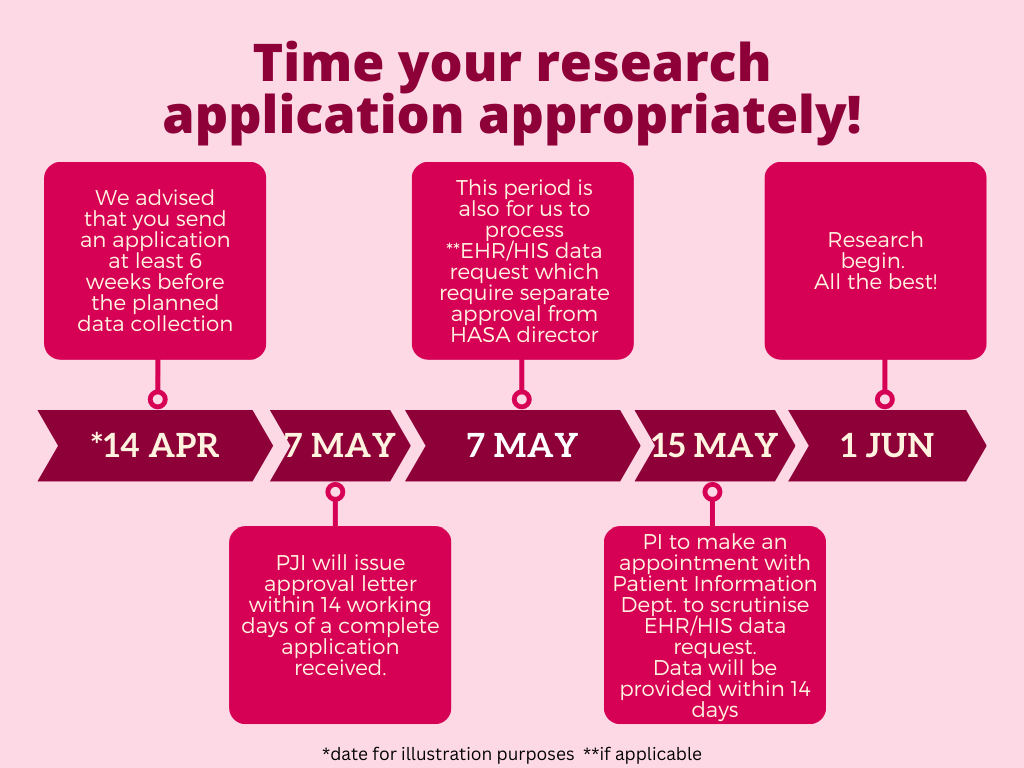
HASA is proud to be using its internally developed in-house EHR called UniMEDS. In line with the spirit to empower and elevate research culture as a whole, EHR data may be requested for research purposes such as for conducting secondary data analysis. However, in view of security, privacy, and confidentiality concern, open access to UniMEDS cannot be provided to the researcher. Instead, a researcher is required to fill in the EHR data extraction request form Maklumat parameter bagi pengumpulan data [(500-PJI (18/4/28)/Borang (03)] and detail out the data of interest using the format supplied. Final approval will be obtained from HASA director.
PJI's role is to assist and connect the researcher to the Patient Information department and upon receiving the approval letter to conduct research in HASA, the researcher may contact the Patient Information department directlly to schedule an appointment.
Do take note that UniMEDS is under the custodian of HASA’s Patient Information department and as such they reserve the right and the final say on the type of data allowed or can be granted to the researcher. Please allow for up to 14 days for the data to be extracted and compiled for your usage.
Please be reminded that researcher from UiTM is required to sign the Confidentiality Letter of Undertaking while a non-UiTM researcher must sign a Non-Disclosure Agreement (NDA) with the University for this purpose.
Dana Clinical Excellence Grant (DCEG)
DCEG is HASA's maiden research grant available to all clinical staff in HASA.
The grant was created to bolster and encourage clinical research activities in the hospital.
Congratulations to the previous (first) cycle recipients in 2021.
Stay tuned for the next grant opening.

Research Processes in HASA.
Research Progress Report.
Research under investigator-initiated research (IIR) is required to submit a research report biannually every 6 months (before 31st May and/or 30th November) until the completion of the research. For the last 6 months of the research period, the researcher may submit an End of Research Report (see below) instead of Research Progress Report to conclude his or her research in HASA.
End of Research Report.
Research under investigator-initiated research (IIR) is required to submit an End of Research Report upon the completion of the data collection stage in HASA.
For example, in the case of ongoing research started in July 2022 and schedule to finish in April 2023, the researcher is required to submit Research Progress Report before 30th November 2022 and then the End of Research Report in April 2023 or latest before 31st May 2023.
Publication or Presentation using research findings or data from HASA.
A researcher is required to fill in the Approval Form for Presentation or Publication Submission Using Research Data from HASA with the accompanying drafted publication materials to PJI prior to submission to the organiser/publisher/journal/company. A clear and explicit acknowledgement to HASA must be expressed and a copy of the publication must be provided to PJI for internal reference.
 |
: | DR. MUZZAMMIL MOHD KHAIRI |
| : | MEDICAL OFFICER (UD52) | |
| : | 03-3396 3000 (ext: 14507) |
|
| : | muzzammilkhairi@uitm.edu.my |
 |
: | CIK NOOR MALIZA RAMLI |
| : | CLERK (N19) | |
| : | 03-3396 3000 (ext: 14517) |
|
| : | malizaramli@uitm.edu.my |
INDUSTRIAL LINKAGES
Industrial LinkagesAl-Sultan AbdullahHospital| UiTM

 |
: | PN. KHAZREEN KAMALLUDIN |
| : | ASSISTANT REGISTRAR (N41) | |
| : | 03-3396 3000 (ext: 14509) |
|
| : | khazr635@uitm.edu.my |
 |
: | PN. ERMA IZZATI AMIR |
| : | EXECUTIVE OFFICER (N29) | |
| : | 03-3396 3000 (ext: 3500) |
|
| : | izzati7754@uitm.edu.my |
 |
: | PN. NURUL HUSNA YUSAIDI |
| : | CLERK (N19) | |
| : | 03-3396 3000 (ext: 14517) |
|
| : | husnayusaidi@uitm.edu.my |
INNOVATION AT HASA UiTM
InnovationAl-Sultan AbdullahHospital| UiTM

The completed template with evidence (documentation) can be uploaded to the Google Drive provided below.
 |
: | PN. NUR SYAKILA MOHD ZAKI |
| : | SCIENCE OFFICER (C41) | |
| : | 03-3396 3000 (ext: 14518) |
|
| : | nursya8757@uitm.edu.my |
 |
: | PN. NURUL HUSNA YUSAIDI |
| : | CLERK (N19) | |
| : | 03-3396 3000 (ext: 14517) |
|
| : | husnayusaidi@uitm.edu.my |